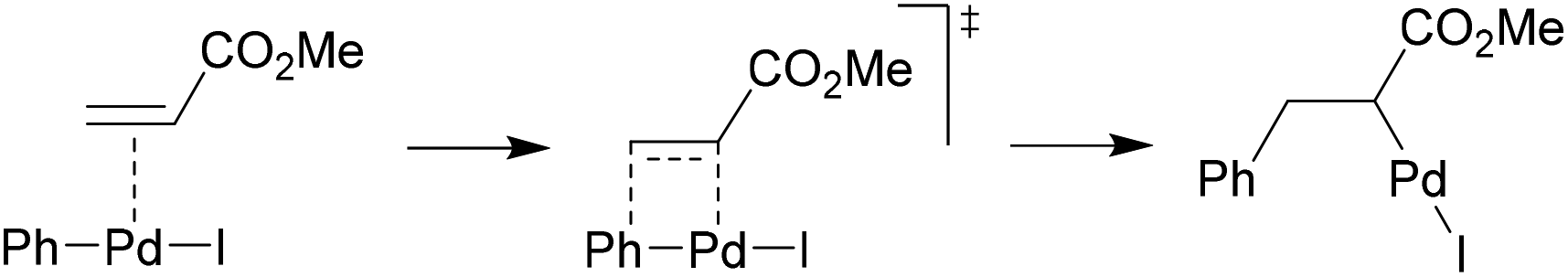
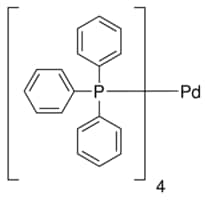
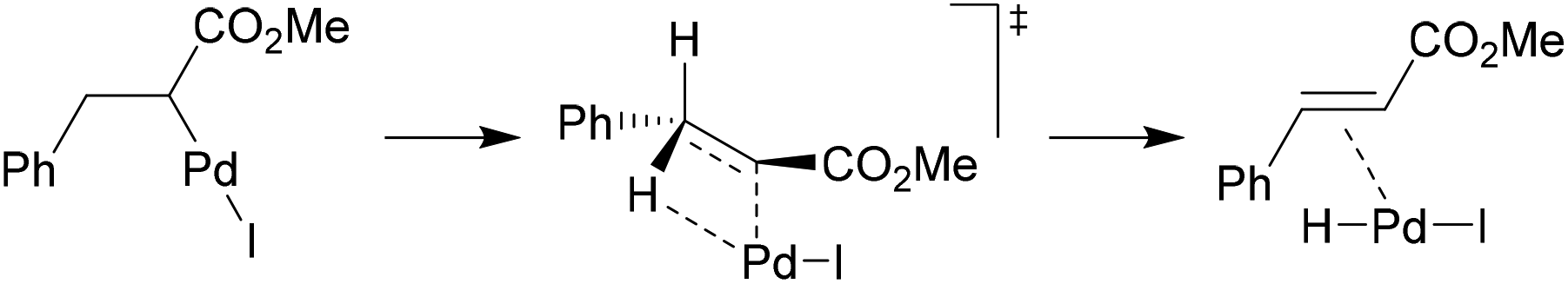
Crystal structure of a novel polymorph of trans-dichlorobis ( triphenylphosphine) palladium (II) and its application as a novel, efficient and retrievable catalyst for the amination of aryl halides and stille cross-coupling reactions -
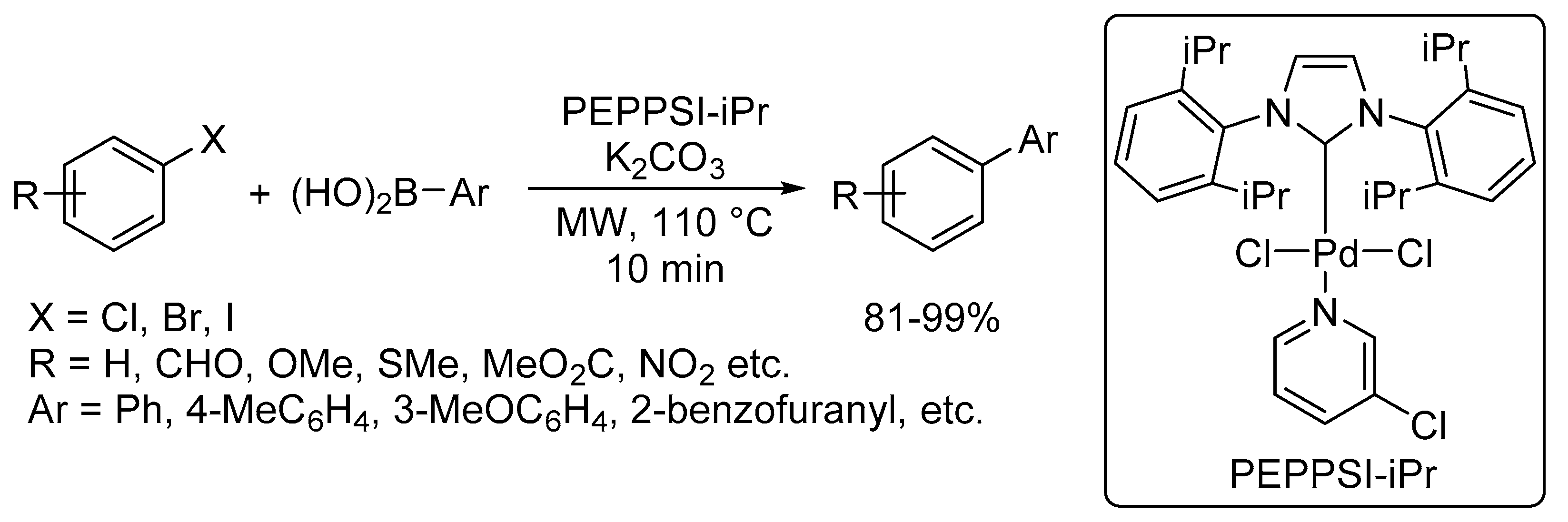
Catalysts | Free Full-Text | Microwave-Assisted Palladium-Catalyzed Cross-Coupling Reactions: Generation of Carbon–Carbon Bond | HTML
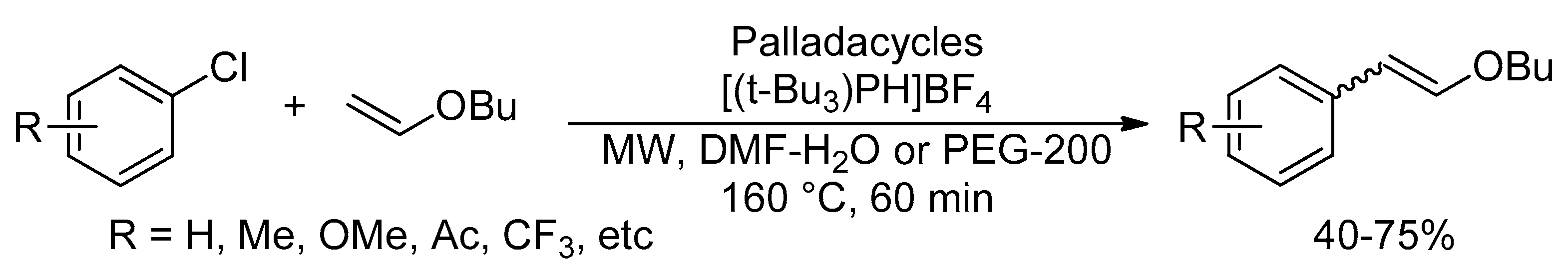
Catalysts | Free Full-Text | Microwave-Assisted Palladium-Catalyzed Cross-Coupling Reactions: Generation of Carbon–Carbon Bond | HTML

Hydrazine‐Free Facile Synthesis of Palladium‐Tetrakis(Triphenylphosphine) - Carrasco - 2019 - European Journal of Inorganic Chemistry - Wiley Online Library
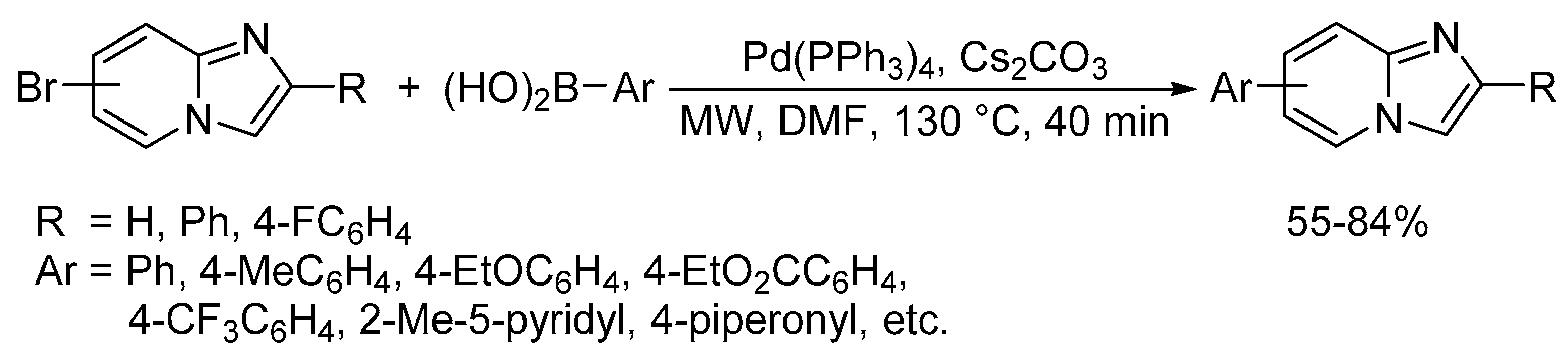
Catalysts | Free Full-Text | Microwave-Assisted Palladium-Catalyzed Cross-Coupling Reactions: Generation of Carbon–Carbon Bond | HTML
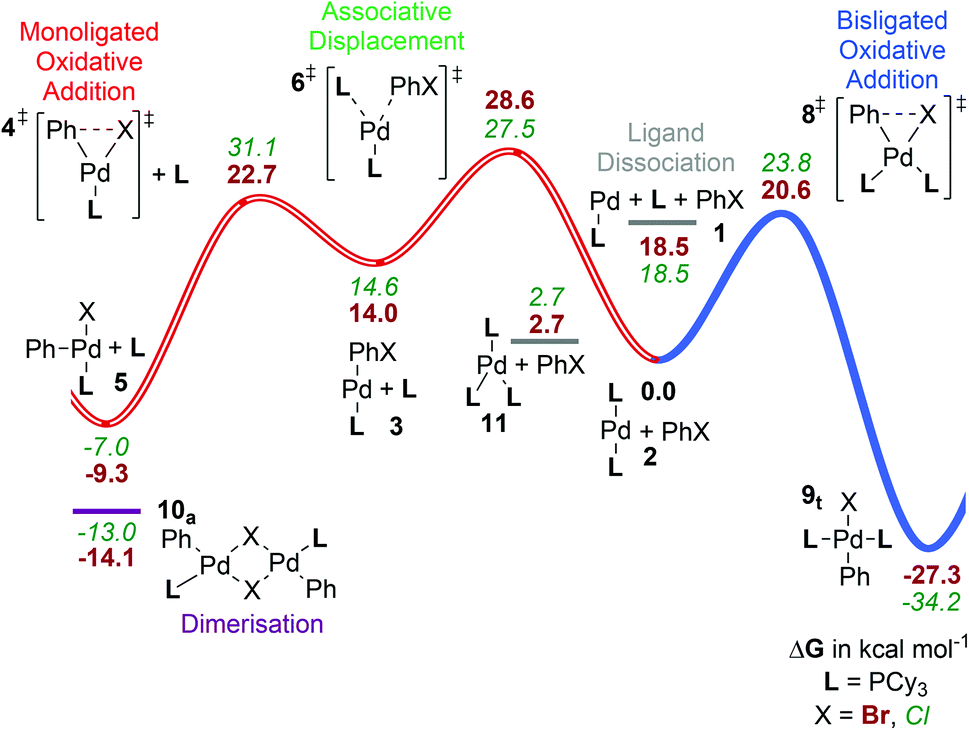
Computed ligand effects on the oxidative addition of phenyl halides to phosphine supported palladium(0) catalysts - Dalton Transactions (RSC Publishing)
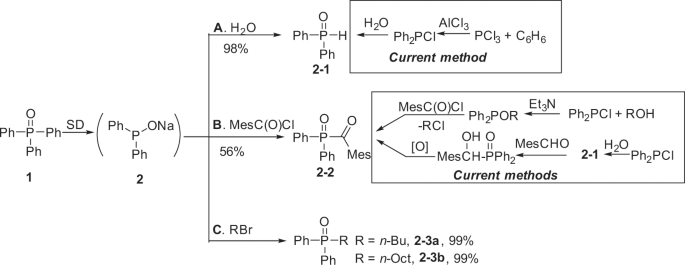
Conversion of triphenylphosphine oxide to organophosphorus via selective cleavage of C-P, O-P, and C-H bonds with sodium | Communications Chemistry

The Palladium Acetate‐Catalyzed Microwave‐Assisted Hirao Reaction without an Added Phosphorus Ligand as a “Green” Protocol: A Quantum Chemical Study on the Mechanism - Keglevich - 2017 - Advanced Synthesis & Catalysis -

Reaction rate between allyl alcohol and Pd(TPPTS) 3 : H + /Pd ratio... | Download Scientific Diagram
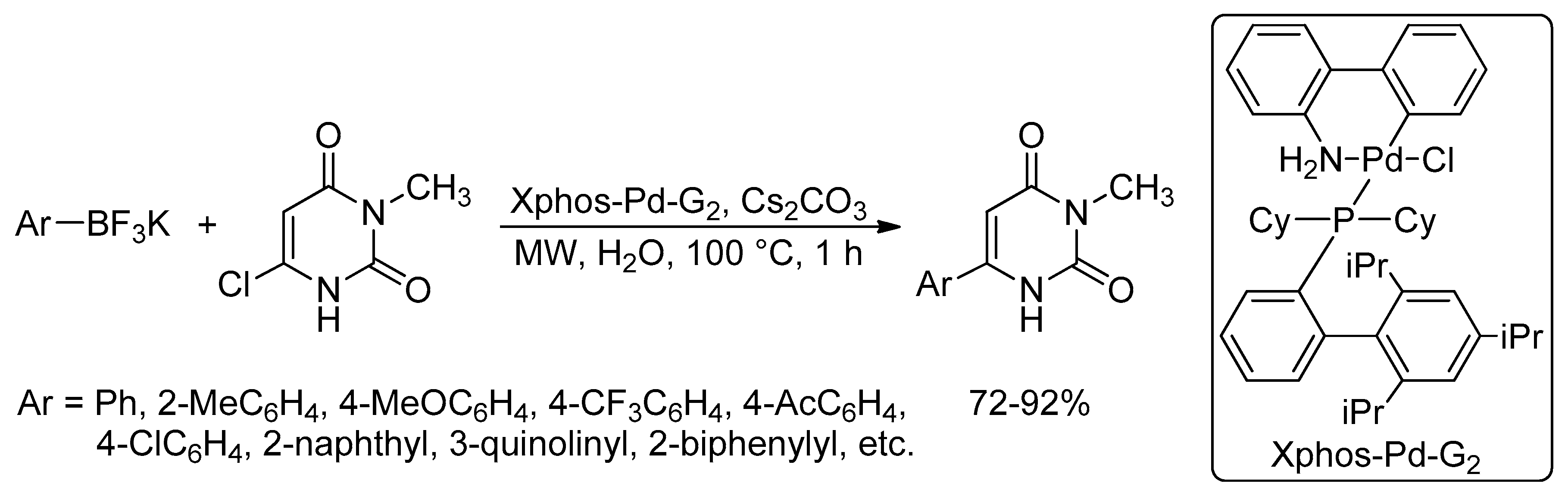
Catalysts | Free Full-Text | Microwave-Assisted Palladium-Catalyzed Cross-Coupling Reactions: Generation of Carbon–Carbon Bond | HTML

Catalysts | Free Full-Text | Microwave-Assisted Palladium-Catalyzed Cross-Coupling Reactions: Generation of Carbon–Carbon Bond | HTML
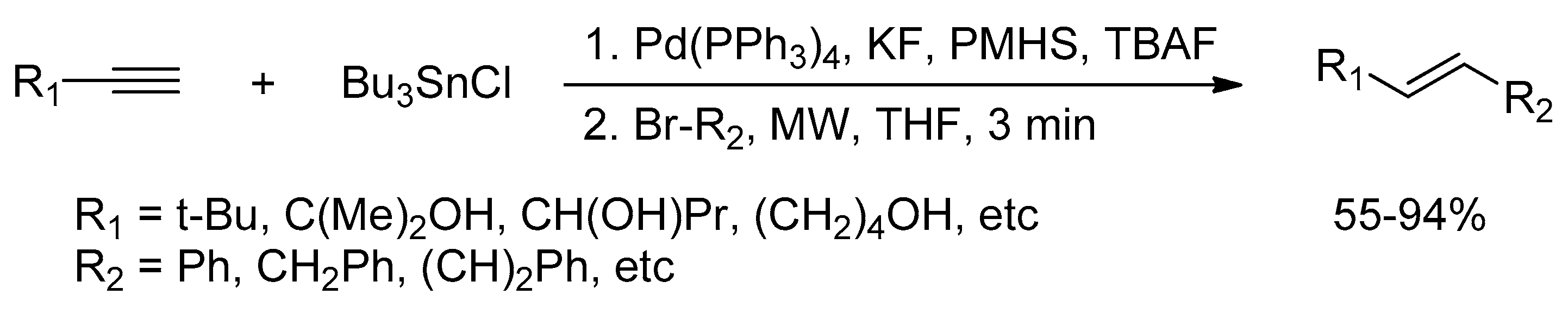
Catalysts | Free Full-Text | Microwave-Assisted Palladium-Catalyzed Cross-Coupling Reactions: Generation of Carbon–Carbon Bond | HTML