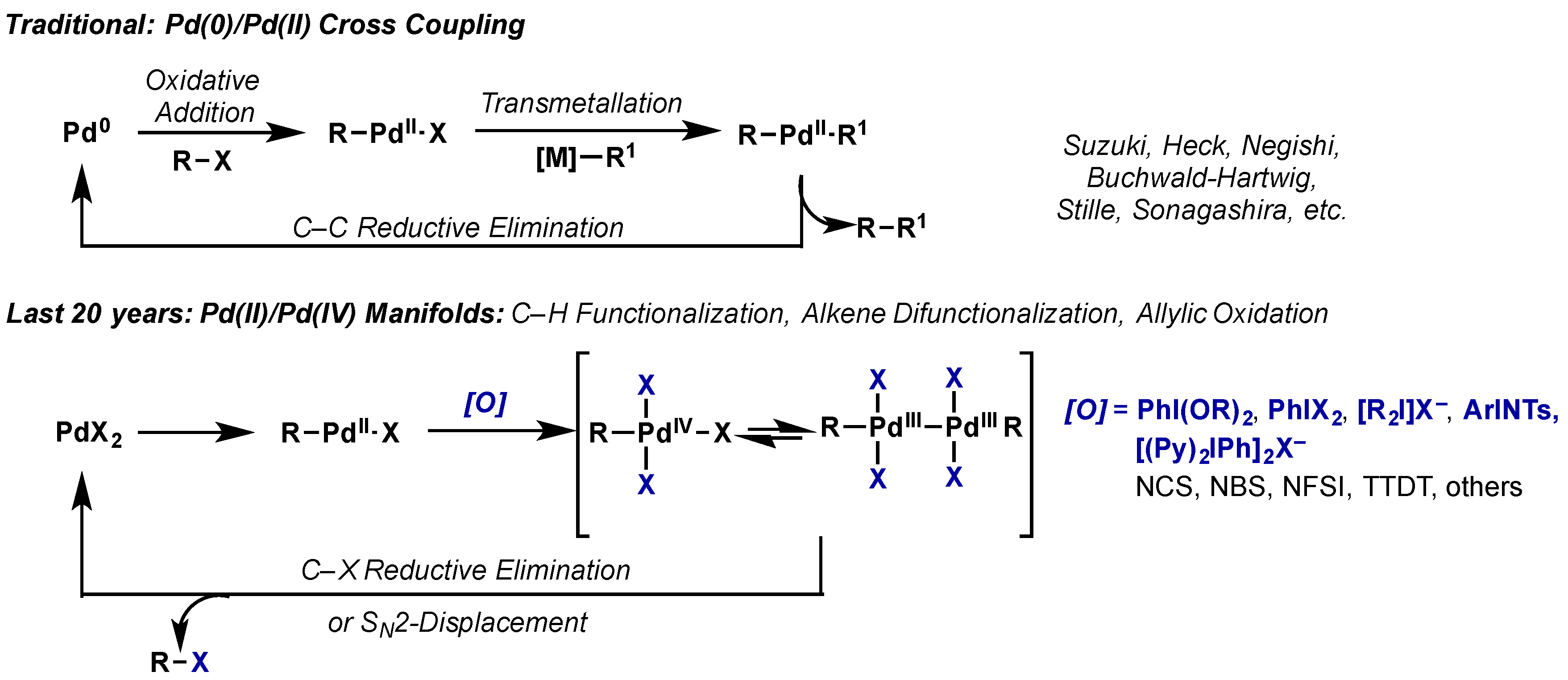
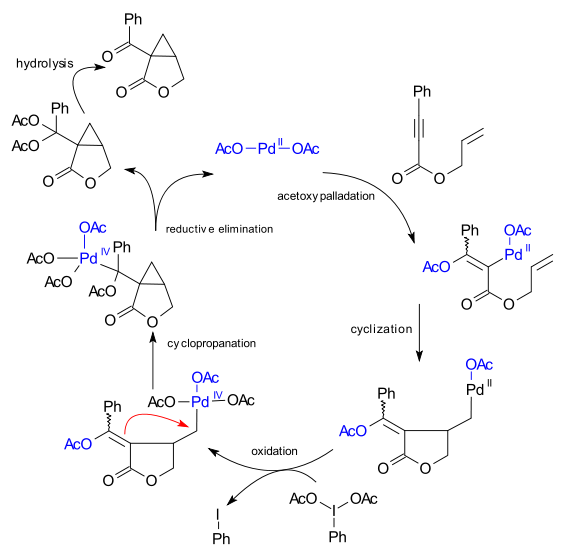
Figure 7 from The mechanism of a ligand-promoted C(sp3)-H activation and arylation reaction via palladium catalysis: theoretical demonstration of a Pd(II)/Pd(IV) redox manifold. | Semantic Scholar
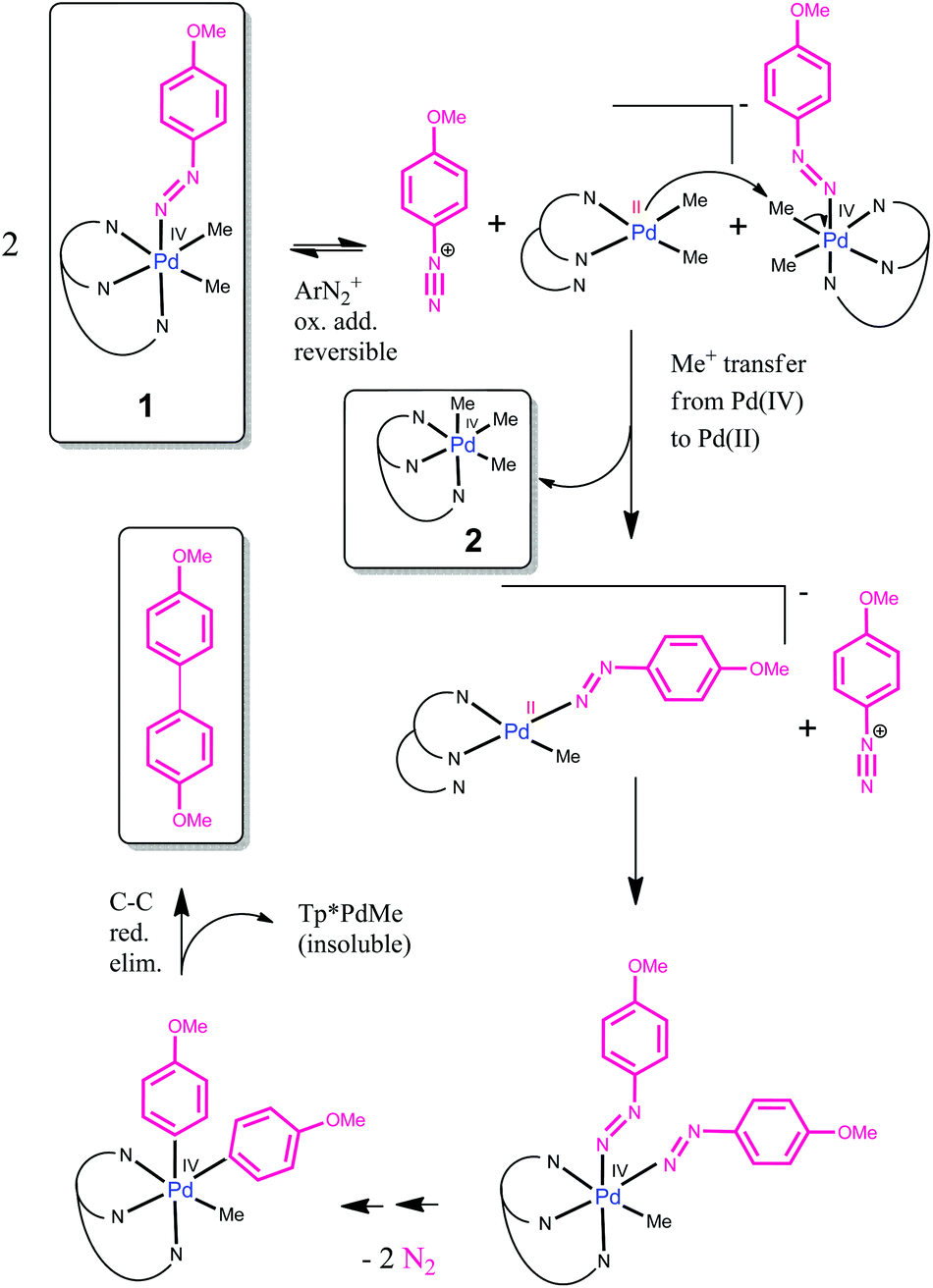
The first palladium( iv ) aryldiazenido complex: relevance for C–C coupling - Dalton Transactions (RSC Publishing) DOI:10.1039/C7DT00078B
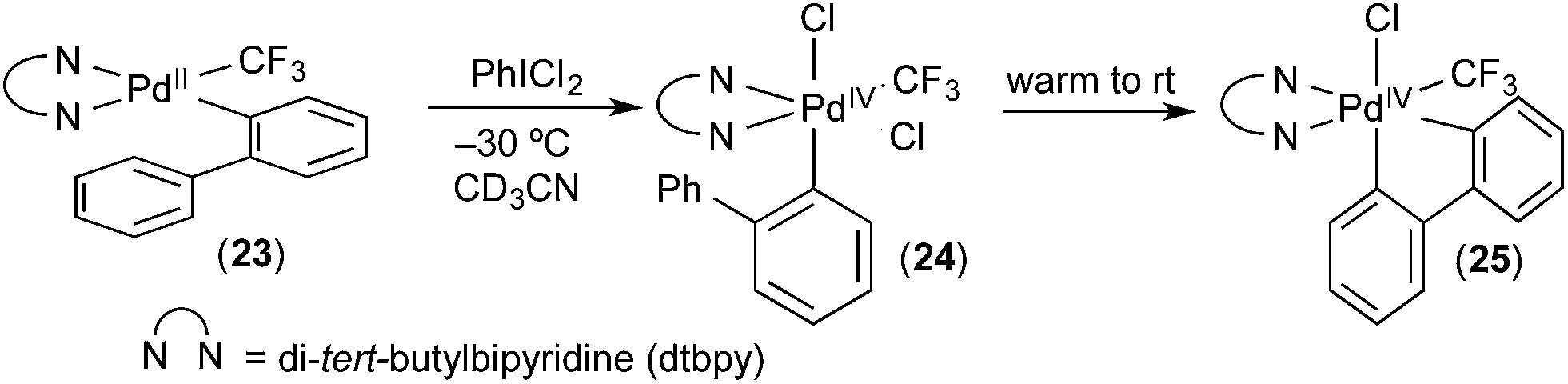
Carbon–hydrogen (C–H) bond activation at Pd IV : a Frontier in C–H functionalization catalysis - Chemical Science (RSC Publishing) DOI:10.1039/C4SC02591A

Controlling Pd( iv ) reductive elimination pathways enables Pd( ii )-catalysed enantioselective C( sp 3 )−H fluorination | Nature Chemistry

The mechanism of palladium(II)-mediated C–H cleavage with mono-N-protected amino acid (MPAA) ligands: origins of rate acceleration in: Pure and Applied Chemistry Volume 88 Issue 1-2 (2016)

Figure 1 from The mechanism of a ligand-promoted C(sp3)-H activation and arylation reaction via palladium catalysis: theoretical demonstration of a Pd(II)/Pd(IV) redox manifold. | Semantic Scholar

Figure 3 from The mechanism of a ligand-promoted C(sp3)-H activation and arylation reaction via palladium catalysis: theoretical demonstration of a Pd(II)/Pd(IV) redox manifold. | Semantic Scholar

Insight into the palladium-catalyzed oxidative arylation of benzofuran: heteropoly acid oxidants evoke a Pd(II)/Pd(IV) mechanism - ScienceDirect

Figure 10 from Mechanism of C-F reductive elimination from palladium(IV) fluorides. | Semantic Scholar
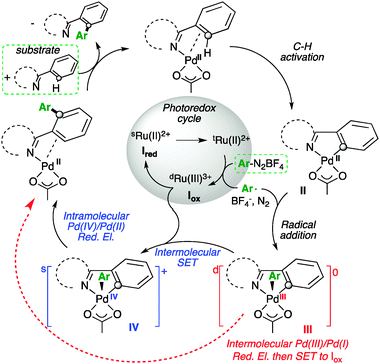
Radical Pd(iii)/Pd(i) reductive elimination in palladium sequences - Chemical Communications (RSC Publishing)

Selective Reductive Elimination at Alkyl Palladium(IV) by Dissociative Ligand Ionization: Catalytic C(sp3)−H Amination to Azetidines - Nappi - 2018 - Angewandte Chemie International Edition - Wiley Online Library
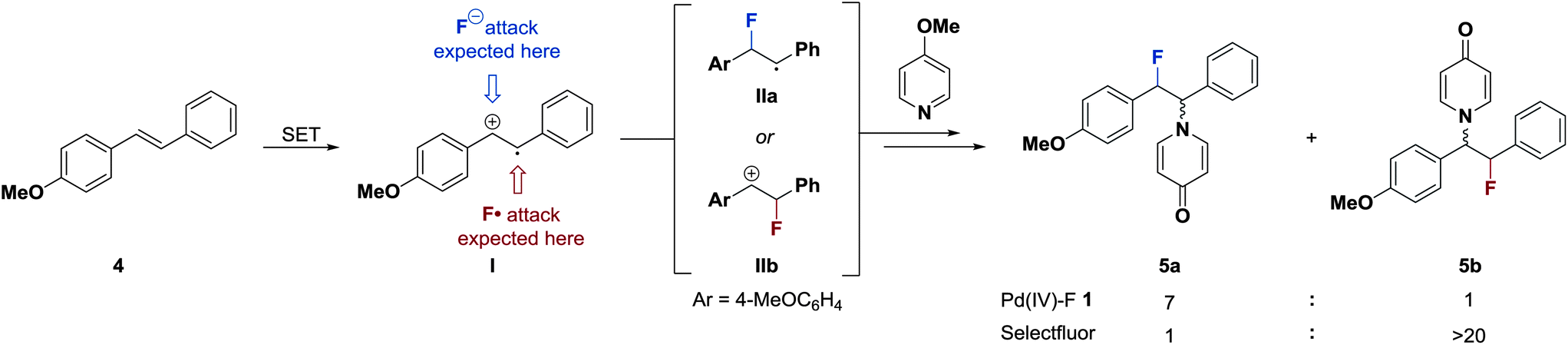
Mechanism of electrophilic fluorination with Pd(iv): fluoride capture and subsequent oxidative fluoride transfer - Chemical Science (RSC Publishing)

Figure 10 from Mechanism of C-F reductive elimination from palladium(IV) fluorides. | Semantic Scholar

Report: Toward Greater Understanding and Expanded Utility of the Palladium-Catalyzed Activation of Carbon-Carbon Single Bonds (56th Annual Report on Research Under Sponsorship of The American Chemical Society Petroleum Research Fund)

DFT studies of isomerization in palladium(IV) chemistry and alkyl halide transfer from palladium(IV) to palladium(II) - ScienceDirect