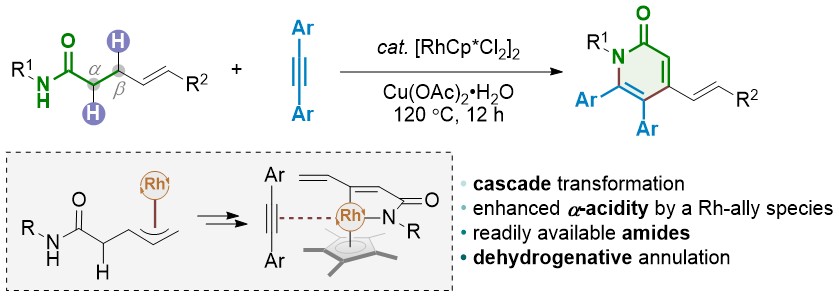
PDF) ChemInform Abstract: Sulfur-Functionalized N-Heterocyclic Carbene Complexes of Pd(II): Syntheses, Structures and Catalytic Activities

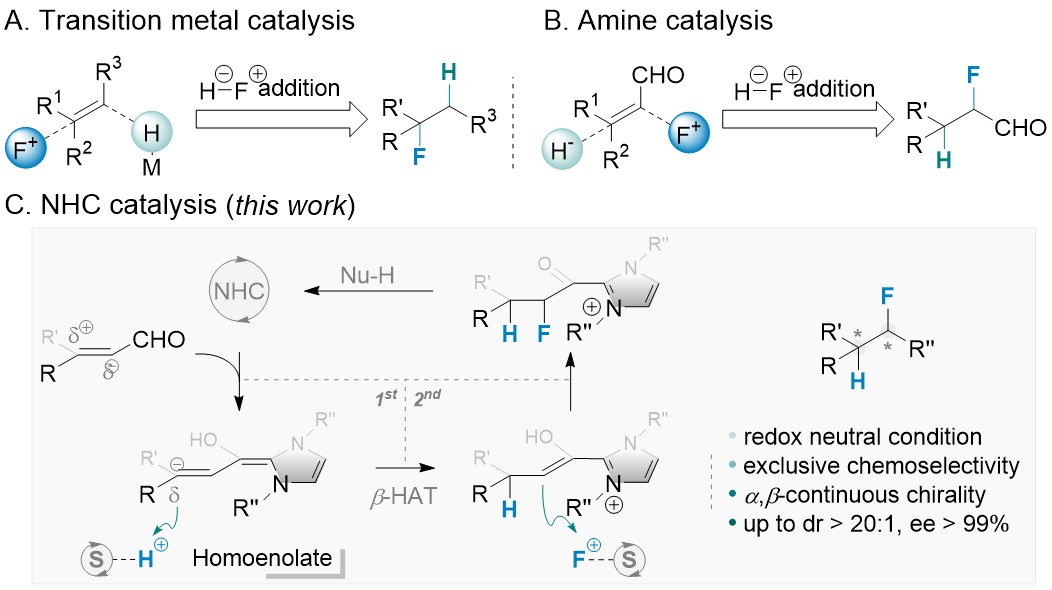
Palladium/N-heterocyclic carbene catalysed regio and diastereoselective reaction of ketones with allyl reagents via inner-sphere mechanism | Nature Communications

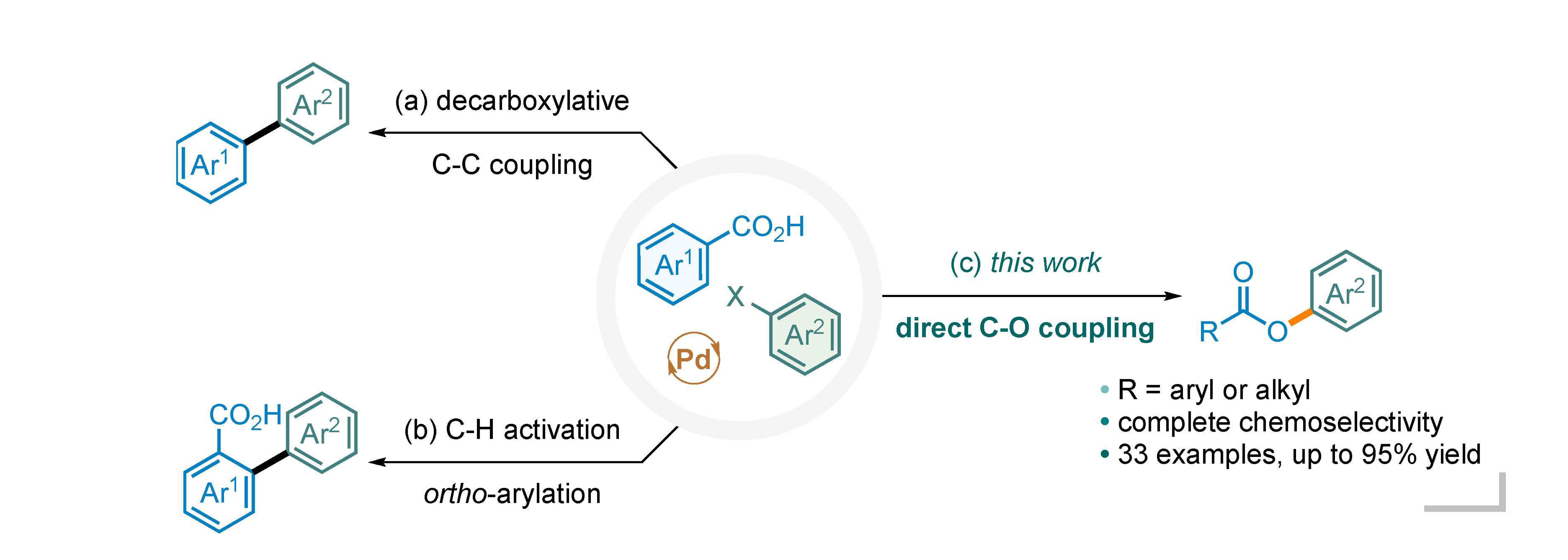
Palladium‐Catalyzed C–S Bond Formation: Rate and Mechanism of the Coupling of Aryl or Vinyl Halides with a Thiol Derived from a Cysteine - Moreau - 2005 - European Journal of Organic Chemistry -

Palladium N-heterocyclic carbene catalyzed regioselective thiolation of 1-aryl-3-methyl-1H-pyrazol-5(4H)-ones using aryl thiols - ScienceDirect

Palladium N-heterocyclic carbene catalyzed regioselective thiolation of 1-aryl-3-methyl-1H-pyrazol-5(4H)-ones using aryl thiols - ScienceDirect

Pd nanoparticles stabilized on the Schiff base-modified boehmite: Catalytic role in Suzuki coupling reaction and reduction of nitroarenes - ScienceDirect

Palladium‐Catalyzed C–S Bond Formation: Rate and Mechanism of the Coupling of Aryl or Vinyl Halides with a Thiol Derived from a Cysteine - Moreau - 2005 - European Journal of Organic Chemistry -

Palladium‐Catalyzed C–S Bond Formation: Rate and Mechanism of the Coupling of Aryl or Vinyl Halides with a Thiol Derived from a Cysteine - Moreau - 2005 - European Journal of Organic Chemistry -

Palladium‐Catalyzed C–S Bond Formation: Rate and Mechanism of the Coupling of Aryl or Vinyl Halides with a Thiol Derived from a Cysteine - Moreau - 2005 - European Journal of Organic Chemistry -

Palladium‐Catalyzed C–S Bond Formation: Rate and Mechanism of the Coupling of Aryl or Vinyl Halides with a Thiol Derived from a Cysteine - Moreau - 2005 - European Journal of Organic Chemistry -

Palladium N-heterocyclic carbene catalyzed regioselective thiolation of 1-aryl-3-methyl-1H-pyrazol-5(4H)-ones using aryl thiols - ScienceDirect
![Pd(NHC)(allyl)Cl] Complexes: Synthesis and Determination of the NHC Percent Buried Volume (%Vbur) Steric Parameter - Clavier - 2009 - European Journal of Inorganic Chemistry - Wiley Online Library Pd(NHC)(allyl)Cl] Complexes: Synthesis and Determination of the NHC Percent Buried Volume (%Vbur) Steric Parameter - Clavier - 2009 - European Journal of Inorganic Chemistry - Wiley Online Library](https://chemistry-europe.onlinelibrary.wiley.com/cms/asset/a8f28041-c485-4e67-919c-7bc032cd2781/mscheme1.jpg)
Pd(NHC)(allyl)Cl] Complexes: Synthesis and Determination of the NHC Percent Buried Volume (%Vbur) Steric Parameter - Clavier - 2009 - European Journal of Inorganic Chemistry - Wiley Online Library

Recent advances in the Suzuki–Miyaura cross-coupling reaction using efficient catalysts in eco-friendly media - Green Chemistry (RSC Publishing) DOI:10.1039/C8GC02860E

An Antitumor Bis(N-Heterocyclic Carbene)Platinum(II) Complex That Engages Asparagine Synthetase as an Anticancer Target. - Angew. Chem. Int. Ed. - X-MOL
New trends in the functionalization of metallic gold: from organosulfur ligands to N-heterocyclic carbenes

Robust gold nanorods stabilized by bidentate N-heterocyclic-carbene–thiolate ligands | Nature Chemistry

Palladium‐Catalyzed C–S Bond Formation: Rate and Mechanism of the Coupling of Aryl or Vinyl Halides with a Thiol Derived from a Cysteine - Moreau - 2005 - European Journal of Organic Chemistry -

Palladium‐Catalyzed C–S Bond Formation: Rate and Mechanism of the Coupling of Aryl or Vinyl Halides with a Thiol Derived from a Cysteine - Moreau - 2005 - European Journal of Organic Chemistry -