![Dansyl-PEG-phenylboronic acid | Protein labeling reagent | CAS [N.A.] | Axon 2257 | Axon Ligand™ with >98% purity available from stock from supplier Axon Medchem Dansyl-PEG-phenylboronic acid | Protein labeling reagent | CAS [N.A.] | Axon 2257 | Axon Ligand™ with >98% purity available from stock from supplier Axon Medchem](https://www.axonmedchem.com/media/catalog/product/cache/1/image/9df78eab33525d08d6e5fb8d27136e95/2/2/2257.gif)
Dansyl-PEG-phenylboronic acid | Protein labeling reagent | CAS [N.A.] | Axon 2257 | Axon Ligand™ with >98% purity available from stock from supplier Axon Medchem

Recent Progress in the Use of Pd-Catalyzed C-C Cross-Coupling Reactions in the Synthesis of Pharmaceutical Compounds
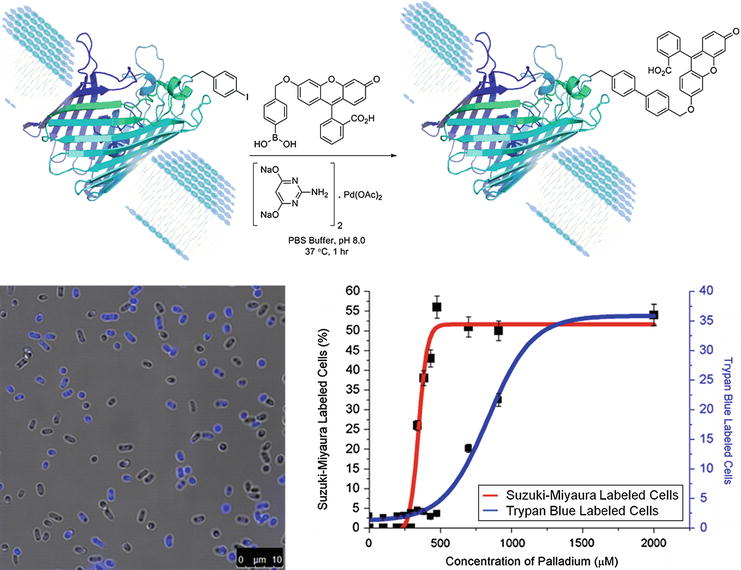
Catalysts | Free Full-Text | The Suzuki–Miyaura Cross-Coupling as a Versatile Tool for Peptide Diversification and Cyclization | HTML

Recent Progress in the Use of Pd-Catalyzed C-C Cross-Coupling Reactions in the Synthesis of Pharmaceutical Compounds
Catalysts | Free Full-Text | The Suzuki–Miyaura Cross-Coupling as a Versatile Tool for Peptide Diversification and Cyclization | HTML

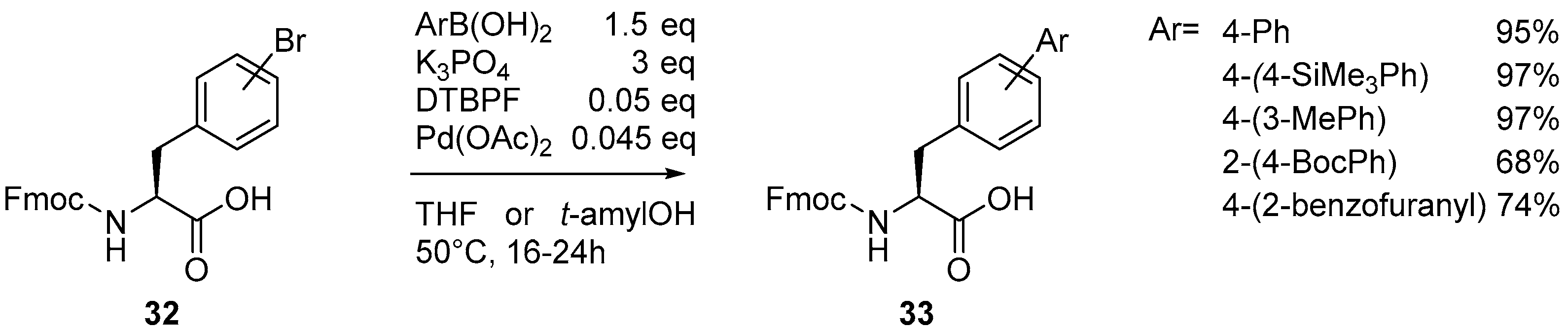
Cationic” Suzuki–Miyaura Coupling with Acutely Base-Sensitive Boronic Acids,Journal of the American Chemical Society - X-MOL

Cross-coupling reaction on Dha:a)General reaction scheme for the Dha... | Download Scientific Diagram

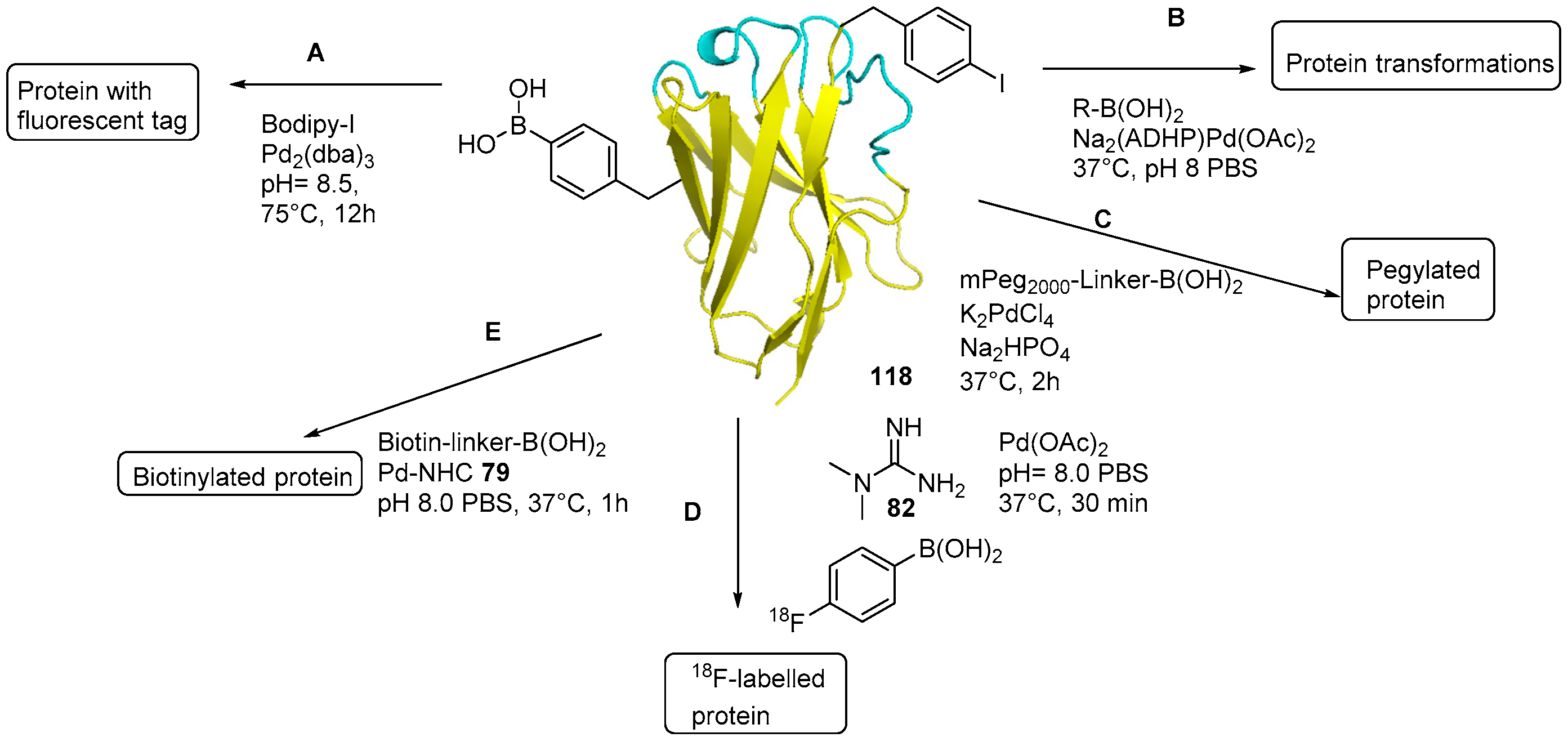
Boronic Acids as Bioorthogonal Probes for Site‐Selective Labeling of Proteins - Akgun - 2018 - Angewandte Chemie International Edition - Wiley Online Library

Boronic Acids as Bioorthogonal Probes for Site‐Selective Labeling of Proteins - Akgun - 2018 - Angewandte Chemie International Edition - Wiley Online Library

Boronic Acids as Bioorthogonal Probes for Site‐Selective Labeling of Proteins - Akgun - 2018 - Angewandte Chemie International Edition - Wiley Online Library
![Boronic Acids and Derivatives | [Synthesis & Materials]Products | Laboratory Chemicals-FUJIFILM Wako Pure Chemical Corporation Boronic Acids and Derivatives | [Synthesis & Materials]Products | Laboratory Chemicals-FUJIFILM Wako Pure Chemical Corporation](https://labchem-wako.fujifilm.com/us/category/images/00002-img03.png)
Boronic Acids and Derivatives | [Synthesis & Materials]Products | Laboratory Chemicals-FUJIFILM Wako Pure Chemical Corporation

Boronic Acids as Bioorthogonal Probes for Site‐Selective Labeling of Proteins - Akgun - 2018 - Angewandte Chemie International Edition - Wiley Online Library

Palladium-catalyzed Suzuki–Miyaura coupling of amides by carbon–nitrogen cleavage: general strategy for amide N–C bond activation - Organic & Biomolecular Chemistry (RSC Publishing)