Transition-metal-free C–C bond forming reactions of aryl, alkenyl and alkynylboronic acids and their derivatives - Chemical Society Reviews (RSC Publishing)
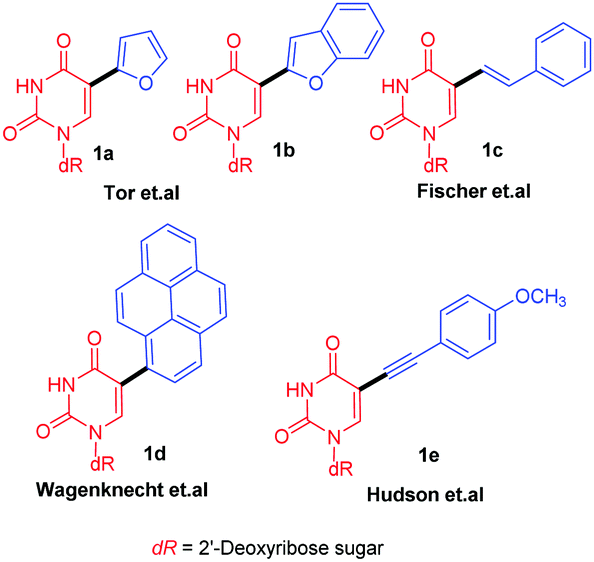
Extended fluorescent uridine analogues: synthesis, photophysical properties and selective interaction with BSA protein - New Journal of Chemistry (RSC Publishing)

Recent advances in the cross-coupling reactions of organoboron derivatives with organic electrophiles, 1995–1998

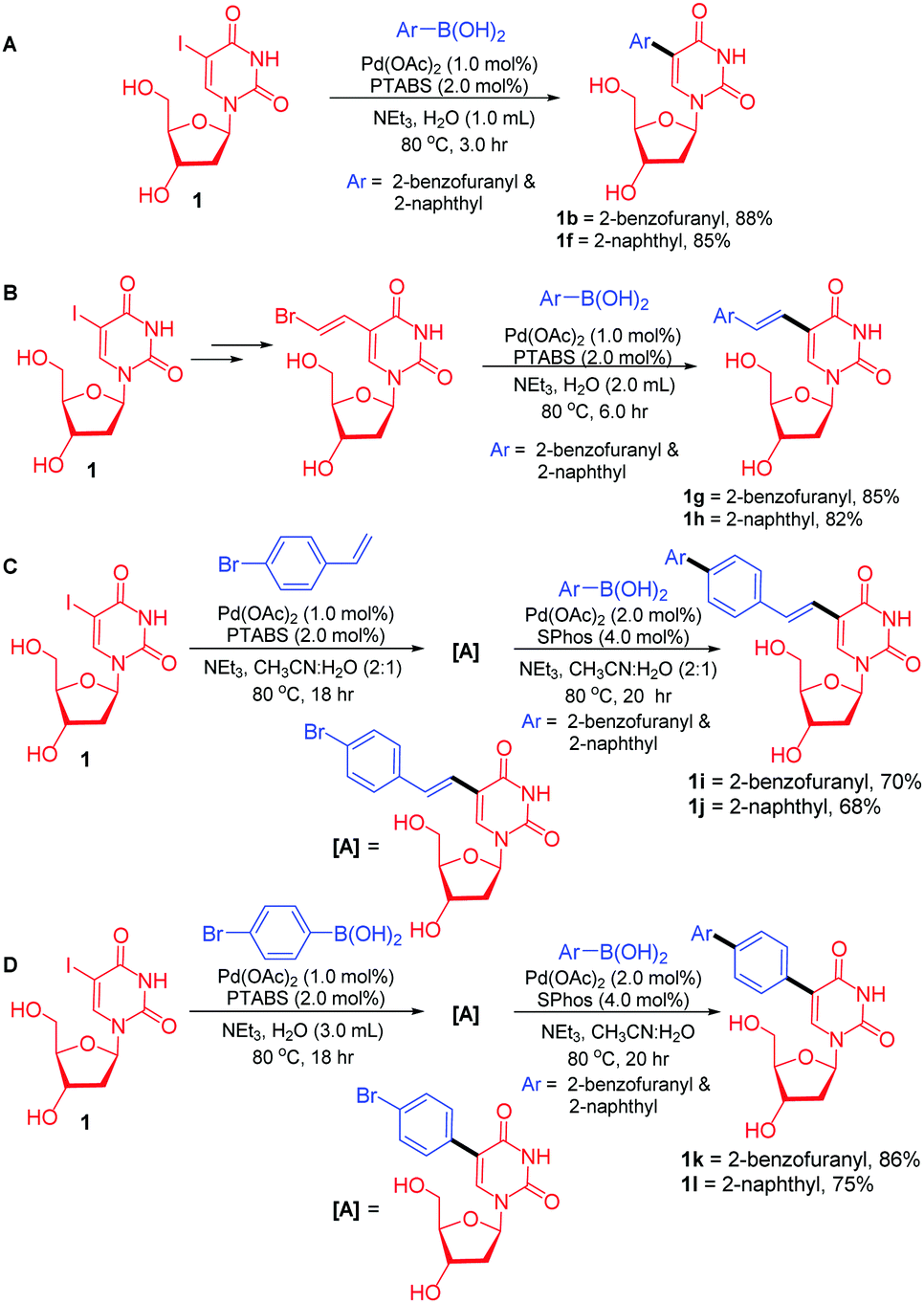
Novel water-soluble phosphatriazenes: versatile ligands for Suzuki–Miyaura, Sonogashira and Heck reactions of nucleosides - RSC Advances (RSC Publishing)

Transition-metal-free C–C bond forming reactions of aryl, alkenyl and alkynylboronic acids and their derivatives - Chemical Society Reviews (RSC Publishing)

Novel water-soluble phosphatriazenes: versatile ligands for Suzuki–Miyaura, Sonogashira and Heck reactions of nucleosides - RSC Advances (RSC Publishing)

Novel water-soluble phosphatriazenes: versatile ligands for Suzuki–Miyaura, Sonogashira and Heck reactions of nucleosides - RSC Advances (RSC Publishing)
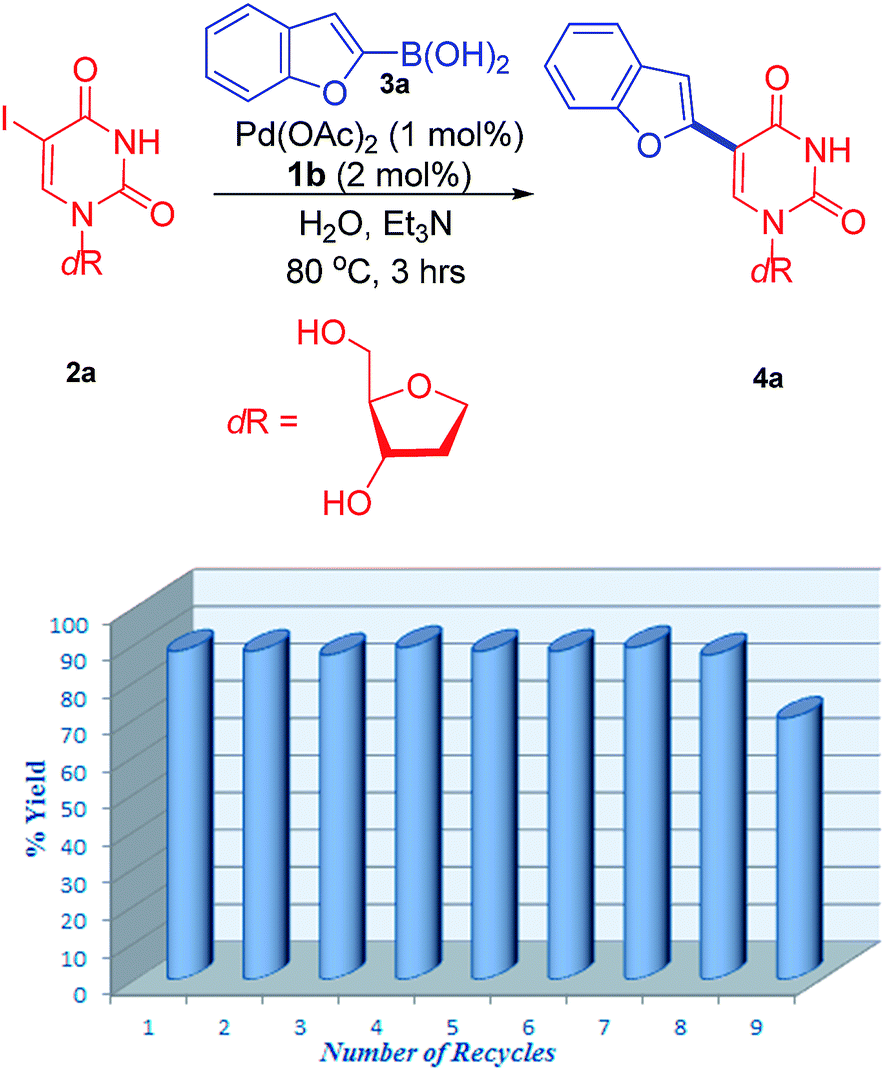
Novel water-soluble phosphatriazenes: versatile ligands for Suzuki–Miyaura, Sonogashira and Heck reactions of nucleosides - RSC Advances (RSC Publishing)

Transition-metal-free C–C bond forming reactions of aryl, alkenyl and alkynylboronic acids and their derivatives - Chemical Society Reviews (RSC Publishing)
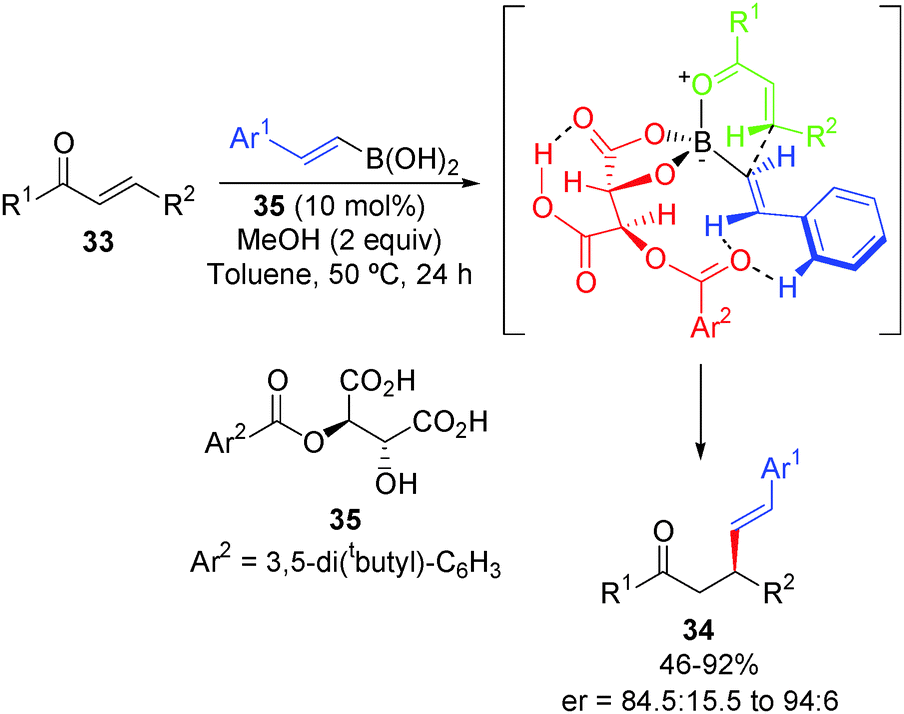
Transition-metal-free C–C bond forming reactions of aryl, alkenyl and alkynylboronic acids and their derivatives - Chemical Society Reviews (RSC Publishing)

Recent advances in the cross-coupling reactions of organoboron derivatives with organic electrophiles, 1995–1998

A simple and highly selective fluorescent sensor for palladium based on benzofuran-2-boronic acid - ScienceDirect

Scope of the Suzuki-Miyaura cross-coupling reactions of potassium heteroaryltrifluoroborates. - Abstract - Europe PMC

Palladium‐Catalyzed Arylation of Arylboronic Acids with Yagupolskii–Umemoto Reagents - Wang - 2016 - Chemistry – A European Journal - Wiley Online Library